
Alejandro Herrera Orrego
CIC biomaGUNE
Developing of an ATP-Independent and Cell-Free Enzymatic Cascade for the Biosynthesis of β-Hydroxy Acids Using Vinyl Esters as Smart Substrates
The synthesis of compounds via in vitro biosynthetic pathways requires both redox power and energy, typically in the form of NAD(P)H and ATP, respectively. Regenerating these cofactors using chemicals, electricity, or light significantly adds to the process’s cost, reducing atom efficiency or increasing energy consumption. In contrast, certain biosystems, such as those involved in β-hydroxy acid production and its polymers, demonstrate in situ cofactor regeneration. These systems commonly involve CoA-dependent Claisen condensation and NAD(P)H-dependent asymmetric reduction steps. In vivo experiments have achieved production levels of up to 3 g/L in these systems [1]. However, in vitro systems have struggled to reach more than 40 mM of poly(3-hydroxy butyrate) [2]. Alternatively, artificial cell-free circular metabolisms, encompassing these reactions, are emerging to convert CO2 into acyl-CoA, yielding C2-C3 compounds in the µM range [3].
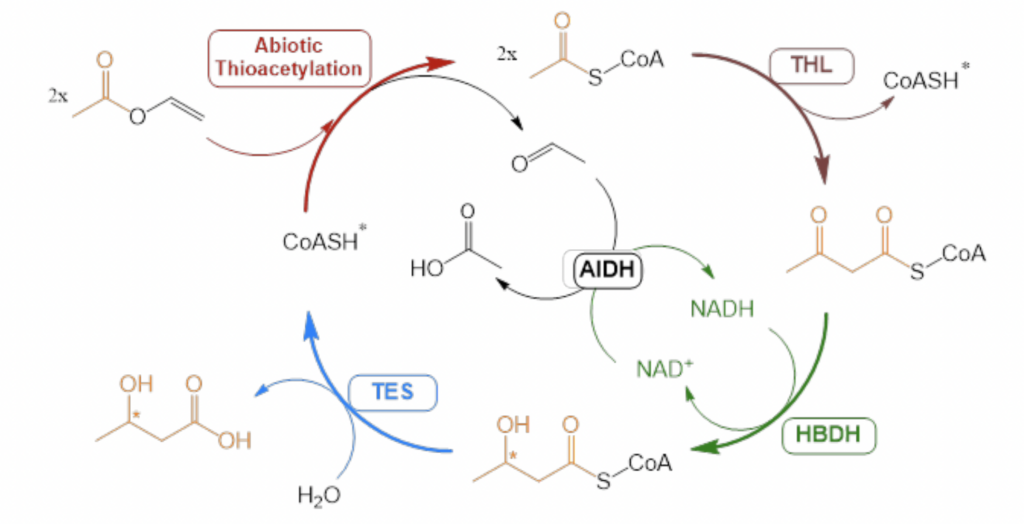
This study proposes a novel method for β-hydroxy acid production through an ATP-independent cascade [4], utilizing vinyl esters as dual acyl and electron donors. This approach embeds chemical energy into the substrate to activate the acyl group and provide redox power. The enzymatic cascade, designed in four steps including abiotic thiolysis, non-decarboxylative Claisen condensation, asymmetric reduction, and hydrolysis with NADH recycling, is catalyzed by four enzymes. This system achieves a titer of 3-hydroxy butyrate of 24 mM without requiring ATP, demonstrating the potential of in vitro biocatalysis to transform simple molecules into multifunctional ones.
 __________
__________
[1] H.-C. Tseng, C. H. Martin, D. R. Nielsen, K. L. J. Prather, Applied and environmental microbiology 2009, 75, 3137-3145. bS. Cheong, J. M. Clomburg, R. Gonzalez, Nature biotechnology 2016, 34, 556-561.
[2] F. Li, X. Wei, L. Zhang, C. Liu, C. You, Z. Zhu, Angewandte Chemie 2022, 134, e202111054.
[3] aS. Sundaram, C. Diehl, N. S. Cortina, J. Bamberger, N. Paczia, T. J. Erb, Angewandte Chemie International Edition 2021, 60, 16420-16425. bT. Schwander, L. Schada von Borzyskowski, S. Burgener, N. S. Cortina, T. J. Erb, Science 2016, 354, 900-904.
[4] A. H. Orrego, M. G. Rubanu, I. L. López, D. Andrés-Sanz, G. García-Marquina, G. E. Pieslinger, L. Salassa, F. López-Gallego, Angew. Chem. Int. Ed. 2023, 62, e202218312; Angew. Chem. 2023, 135, e202218312.
_________
View Abstract as PDF:
_________
Mon. April 15 | 15:10 – 15:30 hrs – Development of a cell-free enzymatic cascade for the synthesis of GDP-fucose – modeling and optimization
