
Claudia Ferrer-Carbonell
Delft University of Technology
Enzymatic cascade to produce (R)-citronellal using an Old Yellow Enzyme and a Short-Chain Dehydrogenase
(R)-Citronellal, a terpene prised for its distinct lemon, rose, and citronella odours, serves as a precursor for (–)-menthol.[1] This chiral monoterpene alcohol is highly valued in the chemical sector for its cooling properties and minty fragrance.[2] Nowadays, the production pathways for (–)-menthol involve (R)-citronellal as an intermediate, together with metal catalysts such as Ru and Pt.[3]
In contrast, flavin mononucleotide (FMN)-containing ene-reductases, specifically from the Old Yellow Enzyme family (OYE; EC 1.6.99.1), have proven to be effective biocatalysts for the reduction reactions of citral, demonstrating asymmetric bioreduction capabilities for a broad range of α,β-unsaturated compounds.[4] Notably, the OYE NCR from Zymomonas mobilis was engineered to alter its stereoselective reduction of citral, to obtain (R)-citronellal instead of the (S)-enantiomer, albeit with 88% ee.[5] Alternatively, OYE2 from Saccharomyces cerevisiae has shown the ability to selectively catalyse the reduction of geranial to (R)-citronellal with 95.5% ee.[6]
This study focuses on the development of a bienzymatic cascade with two consecutive reactions for the (R)-citronellal formation from the inexpensive geraniol (Figure 1). The cascade involves a short-chain dehydrogenase (SDR) catalysing the oxidation of geraniol to geranial, preventing the formation of neral, followed by OYE2 catalysing the reduction to yield (R)-citronellal. Due to potential cross-reactions with the SDR leading to the formation of (R)-citronellol, this cascade requires a two-step biphasic system to provide >95% ee (R)-citronellal.
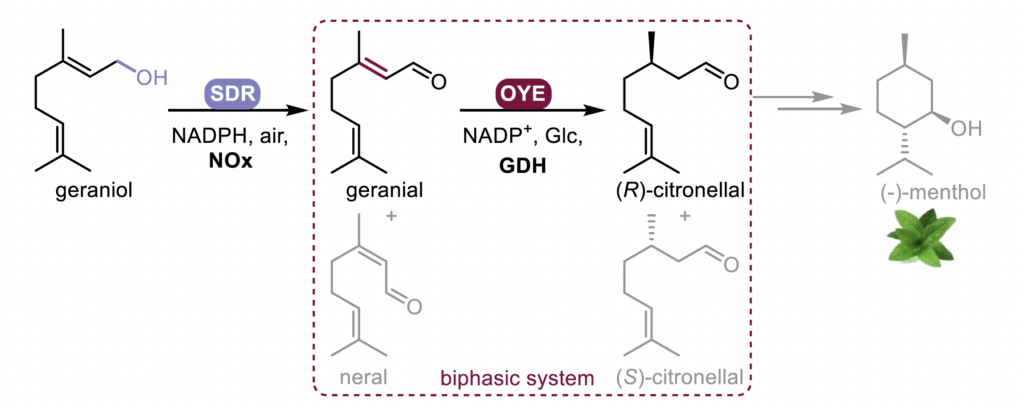
Figure 1.Enzymatic cascade for the production of (R)-citronellal from geraniol.
________
[1] M. Guentert, Flavours and Fragrances 2007, Springer Berlin, Heidelberg.
[2] G. P. Kamatou, I. Vermaak, A. M. Viljoen, B. M. Lawrence, Phytochemistry 2013, 96, 15-25.
[3] M. Azkaar, P. Mäki-Arvela, Z. Vajglová, et al. React. Chem. Eng. 2019, 4, 2156-2169.
[4] Scholtissek, A.; Tischler, D.; Westphal, A. H.; Willem J. H. van Berkel, W. J. H.; Paul, C. E. Catalysts 2017, 7, 130.
[5] Kress, N.; Rapp, J.; Hauer, B. ChemBioChem 2017, 18, 694-823
[6] Ribeaucourt, D.; Höfler, G. T.; Yemloul, M.; Bissaro, B.; Lambert, F.; Berrin, J-G.; Lafond, M.; Paul, C. E. ACS Catal. 2022, 12, 1111-1116.
_________
View Abstract as PDF:
OP15_Claudia_Ferrer-Carbonell.pdf
_________
Wed. April 17 | 10:10 – 10:30 hrs – Enzymatic cascade to produce (R)-citronellal using an Old Yellow Enzyme and a Short-Chain Dehydrogenase
